Laparoscopic Surgical Device Manufacturing
2016-2019 | Process Engineer III
Group Process Development | Applied Medical
At Applied Medical I implement vertically-integrated medical device development projects from design through production phase across three product families. The work involved problem solving using root cause analysis, process capability studies, cross-functional teamwork, and design controls.
In approximately three years I led the 510(k) and CE registration of the Kii Dissecting Balloon (market-disruptive new products for hernia repair), implemented project reducing processing costs by $100,000+ annually, drove customer-derived design modifications to improve device usability, and evaluated customer complaints and initiated projects to improve end user-experience. I also managed a team of three and supervised several interns.
What is Laparoscopic Surgery?
Laparoscopy, often referred to as a minimally invasive option, is an operation performed in the abdomen or pelvis using small incisions with the aid of a camera. The laparoscope aids diagnosis or therapeutic interventions with a few small cuts in the abdomen. Through devices called trocars, various instruments may be inserted to perform a specific task.
Applied Medical prides itself on being a global leader in developing breakthrough technologies and solutions for Minimally Invasive and General Surgery, as well as Bariatric, Cardiac, Colorectal, Gynecologic, Obstetric, Urologic, and Vascular specialties.
What makes Medical Device Different
In the Medical Device Product Life Cycle, all stakeholders must be aware of the stages of a product or project. Despite being focused on process engineering and manufacturing, I was involved in several stages outside of the manufacturing process in order to understand how our processes could be improved.
The heart of medical device regulatory requirements is the ongoing assessment of design inputs and design outputs which may be audited at any time. Design inputs can be thought of the product requirements, they address the intended use of the device as well as the needs of the user (e.g. surgeon) and the patient. Design outputs are evidence that those requirements were met, such as dimensioned drawings of critical components or finalized instructions-for-use. These documents are frequently revisited during most challenged experienced at each stage.
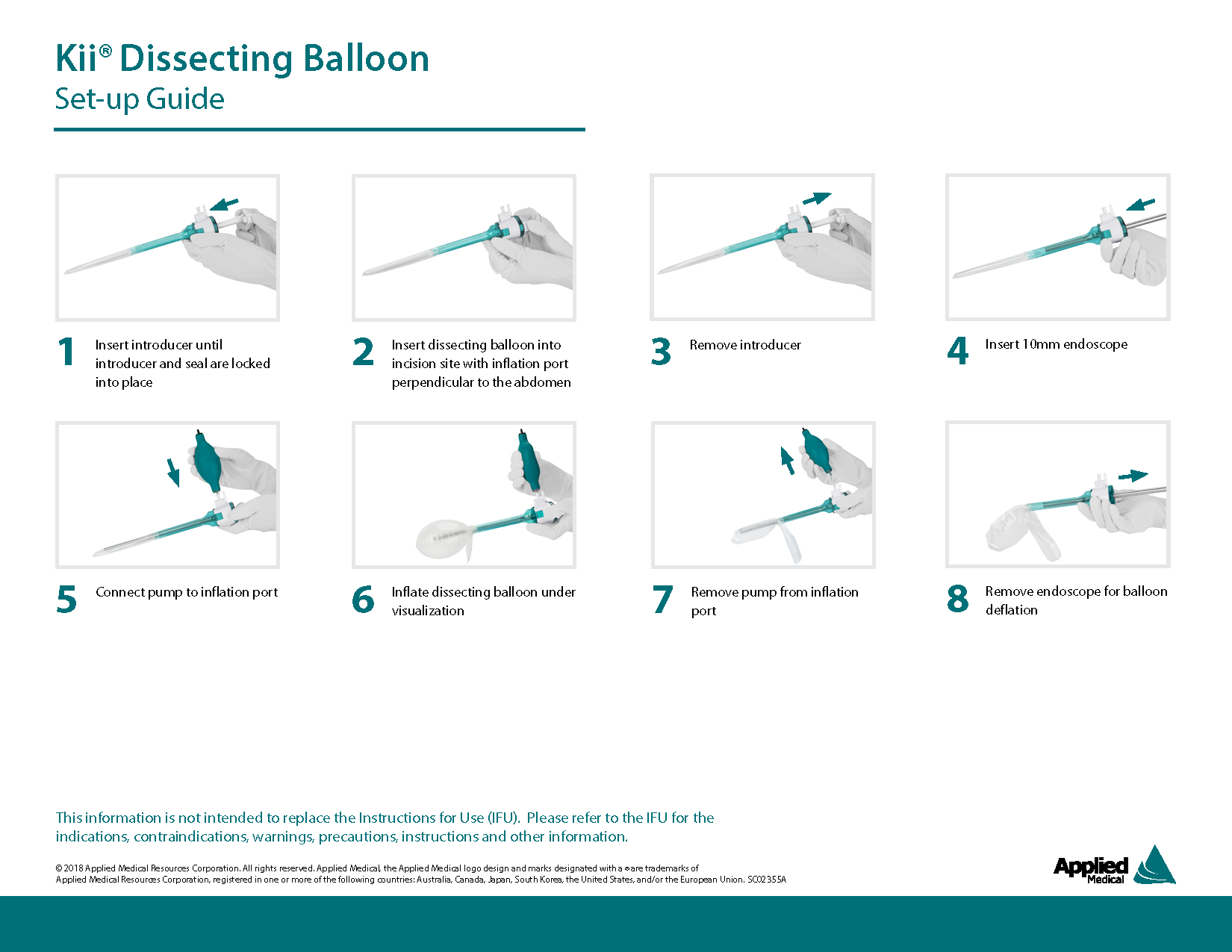
Kii Dissecting Balloon
2017-2019
Inguinal hernia repair is one of the most common operations in general surgery with a rate of 28 in 100,000 persons in the U.S. As part of the totally extra-peritoneal (TEP) hernia repair process, surgeons separate tissue in the abdominal wall in order to improve scope visibility in the cavity. It was discovered that surgeons would separate this tissue by sweeping their gloved finger or another instrument. Various medical device companies acknowledged the risk associated with these methods and developed new means of minimally invasive dissection through the inflation of balloons.
The Challenge
Applied Medical set out to create their own devices for balloon dissection utilizing existing laparoscopic devices manufactured in house, such as the trocar access system. Despite similarities, our team navigated the challenge of re-purposing existing componentry and creating novel parts that enable for optimal TEP hernia dissection. The production and release of these products tested my ability to lead teams, overcome production challenges, and work with regulators.
Design Verification and Validation
The Dissecting Balloon design requirements were primarily validated using statistically significant sample sizes as most destructive testing was not feasible to ensure devices to be sold were functional. I designed and qualified test methods that both assessed the device’s function (specified in the design inputs) and creatively made use of our existing test capabilities. For example, to observe that the balloon was non-fragmenting we simply expand the balloon to pop in a bag, then observe the bag for fragments.
Process Qualification
We were tasked with the development and qualification of the manufacturing and assembly process. I wrote manufacturing instruction guides, taught quality control technicians and assembly operators how to use the equipment, created pass/fail guidelines, and conducted qualification production runs that tested the limits of the process against the intended design (specifications in the design outputs). These devices include plastic injection molding, rubber molding, manual and automated assembly, and sterilization processes all of which occured on site. Our team was responsible for the design of fixtures and tests used in the process. The most critical assembly tests included testing for leak and sheath burst pressure. The devices were qualified for pre-sterilization/post-sterilization transportation and shelf-life testing.
Regulatory Approval
Our team contributed to the 510(k) and CE registration in accordance with ISO 13485 and 21 CFR 820. Simulated use testing is required for FDA approval. We were able to simulate testing through comparison in durometer of abdominal flesh to silicon, approximations of inflation pumps based on human factors data, and addressing similar features in existing products. We collaborated with subject matter experts familiar with hernia repair during cadaver testing.
Post-Market Observation
After launching the product family in the U.S. I was fortunate to observe surgeries with the product in Baltimore, Philadelphia, and Irvine. Viewing the operation provided insights to our process, packaging, and instructional development. Through closing the product life cycle circle with post-market visits, I became better informed about how the product design and development process can be improved, streamlined, and scaled.
Epix Clip Applier
2018-2019
The Epix Clip Applier is a mechanical device which is used to ligate vessels using implantable titanium clips. The most common procedure being a Laparoscopic cholestiechtomy, a surgery to remove the gallbladder. When the device is inserted in a trocar, the distal tip may be rotated 360 degrees for maximum visualization and flexibility. The clips are heat treated to a gold color, except for the last 5 clips which are blue to indicate the device is running low of clips.
Scaling Processes with Care and Reliability
Based on our team’s performance we were asked to support another R&D team improve their manufacturing and inspection processes. The Epix Clip Applier has undergone some criticism due to performance in the field, however, several surgeons swore by its efficacy. Our team worked to streamline the processes, ensure consistency in assembly and manufacturing, and qualify cost-reduction and time-reduction production and sterilization methods.
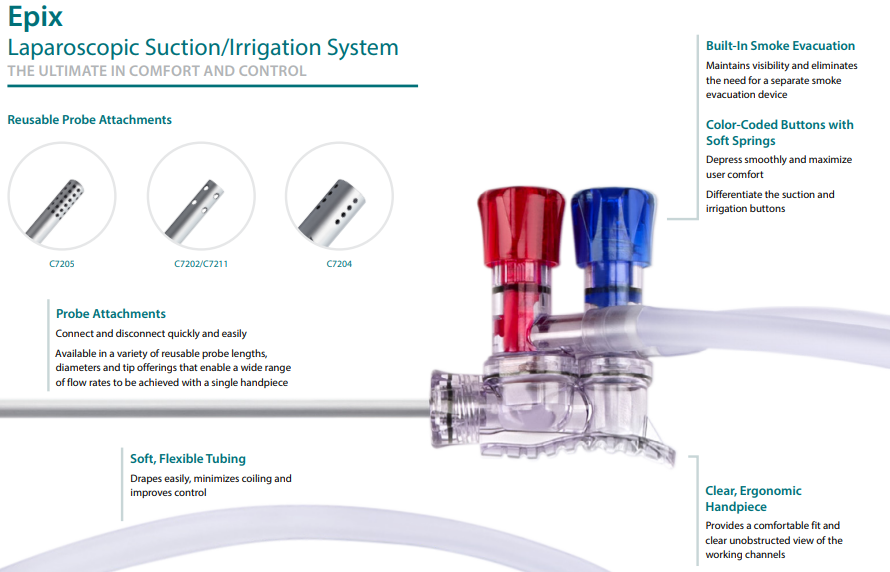
Epix Suction Irrigation
2016-2019
The suction irrigation system is used to clean and remove debris from the surgical cavity. These devices are similar to those used in dentistry which spray water, air, or vacuum fluid. The variations of this system include the probe diameter and tip geometries.
An Evolving Product Family
The Epix Suction Irrigation line was the first product family I had worked on. I learned numerous skills just from the various production processes which include PVC extrusion, CNC swiss screw machining, injection molding, and several small manually assembled components.
The product evolved to have minor design changes of attachment ports, production improvements for consistency and cost reduction, long term projects to change to a non-PVC material for tubing, and new sterilization and packaging projects from Gamma radiation, to E-Beam, and finally to Ethylene Oxide (EO).